Galvanic action occurs when two electrochemically dissimilar metals are in contact and a conductive path occurs for electrons and ions to move from one metal to the other. One metal corrodes as its ions are deposited onto the other metal. Therefore, it is important to keep these dissimilar metals insulated from each other to prevent accelerated corrosion. Generally, water, and especially salt water, serve as the conductive path between two metals so it is important to keep dissimilar metals separated in wet conditions.
Cathodes are noble, or stable, metals that are not prone to corrosion. Examples are gold, silver, nickel, and titanium. Jewelery is made from these materials because it does not corrode rapidly.
Anodes are less stable and are more susceptible to corrosion. Examples include zinc, galvanized steel, and aluminum.
Since anodes and cathodes will react with each other, it is very important to keep these metals apart. For instance, if zinc was in contact with silver and this was submerged in a salt-water solution, the zinc ions would be transferred to the silver and the zinc would corrode.
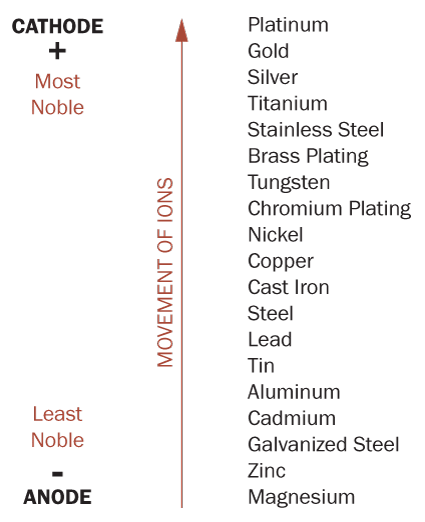
The scale displayed here shows the relative location, in the galvanic series, of many common metals. When two metals are close to each other on the scale, they have a lessor tendency to corrode. However, there are many factors that can affect the corrosion resistance, including the alloy of the metal, and the environment. The galvanic chart shown below is based on the metals being submerged in seawater and should be used only as a general outline.
How to Prevent Metal Corrosion in Architecture
The galvanic scale demonstrates that if a stainless steel element was fastened in place using galvanized (zinc) metal fasteners, the galvanized fasteners would rapidly corrode as its ions move toward the stainless steel, which would cause the stainless steel element to come loose. Needless to say, architects and engineers need to be cautious when selecting fasteners for metal fabrications, especially when moisture may be present.
Fasteners should be made from similar metals that are close to each other on the galvanic chart. Coated fasteners are also an option. We have an article that discussed how metals are coated to protect them from corrosion.
Dissimilar metals should be kept apart with some kind of an insulator like plastic or or rubber. There are also antioxidant pastes that can be used with certain metals like aluminum and copper. Another option is to provide a sacrificial coating or separator that will take the corrosion before the main structural metal is attached.
Whenever possible, only similar metals should be in contact and those metals should be protected from water (especially salt water.)


